Need Help?
Ask an expert
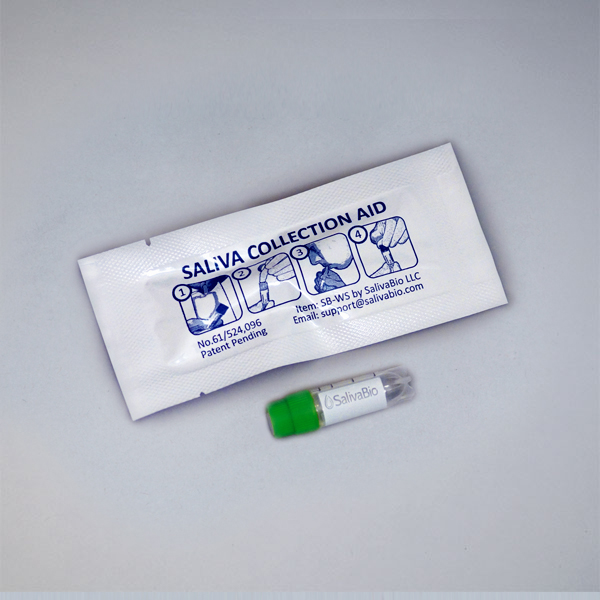
1. How to collect Salivary SARS-CoV-2 (N) IgG
APPROVED SALIVARY SARS-CoV-2 (N-PROTEIN) IgG COLLECTION METHODS
Salivary SARS-CoV-2 (N) IgG Collection Protocol
Collection volume, general considerations, and basic guidelines to maximize salivary SARS-CoV-2 antibody sample integrity. Use this analyte-specific collection protocol to plan you collection methodology and sampling schemes.

2. How to Assay for Salivary SARS-CoV-2 (N) IgG
Send Saliva Samples to Salimetrics
Add to StudyEasy and accurate results from the most trusted Salivary Bioscience Laboratory.
Order Code5225
Salivary COVID-19 (SARS-CoV-2, N) Antibody ELISA Kit
Add to Study
Salimetrics Assay #1-1260
The Salimetrics® SARS-CoV-2 N-protein Salivary IgG ELISA kit is an enzyme-linked immunoassay specifically designed and validated for the qualitative measurement of human IgG specific to the SARS-CoV-2 Nucleocapsid protein (N-protein) in oral fluid which can be utilized in COVID-19 antibody profile research and surveillance testing. This assay kit is not intended for diagnostic use. Research has identified that SARS-CoV-2 antibody testing in saliva could serve as a sensitive and specific serum alternative for large scale immune-surveillance studies. For this assay, the N protein was chosen to maximize the likelihood of antibody detection, since it is the most immunodominant protein in the coronavirus family. Qualitative cutoff values are determined for each run based on controls provided in the kit and serostatus determined from values obtained by oral fluid testing. *Testing for total IgG, using the Salimetrics total IgG ELISA kit, is critical to normalize the sample and also qualify samples for adequate levels of total IgG (above 3 μg/mL) to confidently verify negative samples.* Read More...| Assay Protocol |
|---|
| Rev. 4.30.21
|
| Specifications | |
|---|---|
| Catalog#: | 1-1260 |
| Regulatory Status: | RUO |
| Format: | 96-well plate |
| Assay Time: | ~ 3 hrs |
| Sample Volume/Test: | 50 µL |
| Sensitivity: | 92% |
| Specificity: | 98% |
| Assay Type: | Qualitative |
| Storage Requirements: | 2-8°C |
| Tests Per Kit | |
|---|---|
| Singlet: | 88 |
| Duplicate: | 44 |
| Target Analyte |
|---|
Technical Documentation
Assay Kit Overview
Intended Use
The Salimetrics® SARS-CoV-2 N-Protein Salivary IgG ELISA kit is an enzyme-linked immunoassay specifically designed and validated for the qualitative measurement of human IgG specific to the SARS-CoV-2 Nucleocapsid protein (N-protein) in oral fluid.
It is intended for surveillance testing and not for diagnostic use. This assay kit was optimized and validated for performance in human oral fluid and has not been validated for other human sample types, such as human serum or plasma.
Surveillance testing for antibodies to the SARS-CoV-2 virus can be used to determine if an individual may have been exposed to or infected with this virus, and can be used to understand the percentage of people within a population or community that have developed antibodies to the virus (known as “surveillance tests,” or sero-surveys). (From the FDA guidance document (https://www.fda.gov/media/137599/download))
- When used for surveillance, the results can help determine how widely the virus has spread in communities and how far the pandemic has progressed. Results from tests used for surveillance only are generally not shared with individual patients and are critical for understanding the extent of and risk factors associated with infection.
- Testing individuals may help identify who has developed antibodies against SARS-CoV-2. The results of ongoing research are needed before it is known whether these antibodies are associated with protection from future infection. Current results can help inform who may qualify to donate blood that can be used to manufacture convalescent plasma.
Please read the complete kit insert before performing this assay. Failure to follow kit procedure and recommendations for saliva collection and sample handling may result in unreliable values.
For further information about this kit, its application, or the procedures in this insert, please contact the technical service team at Salimetrics or your local sales representative.
Introduction
The novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was initially identified in the Winter of 2019 with an outbreak designation soon after, at the end of the year (1, 2). The virus rapidly spread achieving global pandemic status in March 2020 and resulted in widespread containment and confinement public health measures in most countries throughout the world.
In addition to virus exposure, there is an urgent need to better understand the levels and duration of protective immunity for public health considerations. The Salimetrics SARS-CoV-2 N-Protein Salivary IgG ELISA assay meets the technical requirement for high sensitivity and specificity performance, while using a saliva sample, allowing for central testing at scale. The main source of salivary IgG antibodies is serum, so it is not surprising that salivary IgG antibodies directly reflect the specificity and activity of those found in serum (3, 4). Oral fluid is, thus, an easily accessible surrogate to serum or plasma in this regard and enables salivary serology studies, surveillance tests or sero-surveys (5). Testing can help determine who has developed antibodies against SARS-CoV-2. Importantly, we have determined that human IgG withstands conditions commonly used for viral heat inactivation (60° or 65°C for 30 min and 95°C for 5 min). The main utility of antibody tests for SARS-CoV-2 supported by the WHO, CDC, FDA and AMA are surveillance studies and the Salimetrics N-Protein IgG ELISA kit meets this need.
Antibody levels decrease in the serum and saliva of COVID-19 patients over time (6-8), and importantly vary depending on disease severity (9, 10). In fact, a higher number of asymptomatic participants become seronegative at 60 days indicating a true decline over a 2-month period rather than as an artifact of assay performance (6, 7). Therefore, our assay is benchmarked on validated commercial serum kit performance instead of molecular tests. Maximizing the likelihood of antibody detection, N protein was chosen as an advantage since it represents the most immunodominant protein in the coronavirus family.
SALIVARY COVID-19 (SARS-CoV-2) IgG ASSAY PRINCIPLE
This is a salivary serological ELISA kit where viral Nucleocapsid antigen is coated on microtiter plates and human IgG antibodies in test saliva samples are detected using an Anti-Human IgG detection antibody linked to horseradish peroxidase (HRP). After each incubation, unbound components are washed away. The levels of IgG bound to the viral antigen are measured by the reaction of the HRP enzyme to the substrate tetramethylbenzidine (TMB). This reaction produces a blue color. A yellow color is formed after stopping the reaction with an acidic solution. The Optical Density is read on a standard plate reader at 450 nm. The total amount of anti-IgG HRP Enzyme Conjugate detected is proportional to the amount of anti-SARS-CoV-2 IgG present in the sample. Normalized Arbitrary Units are calculated by dividing the sample Signal Optical Density by the total IgG concentration of the same sample, determined using the Salimetrics total IgG assay kit, and multiplying this value by 100. The Normalized Arbitrary Units are used to determine if an individual has been infected at least 14 days prior to testing where values above 1.2 are considered positive and below 0.8 negative. Samples reading between these values are considered borderline and a repeat test is recommended for these samples and/or a second collection one or two weeks later.
3. Technical Summary
| Analyte Summary | |
|---|---|
| Analyte: | SARS-CoV-2 Nucleocapsid Protein IgG |
| Aliases: | SARS-CoV-2 IgG, SARS-CoV-2 antibodies, COVID-19 Antibody, 2019-nCoV Antibodies, Coronavirus Antibodies |
| Serum-Saliva Correlation: | NA |
| *Optimum Collection Volume: | 200 μL |
| Assay Summary | |
|---|---|
| Methodology: | ELISA |
| Sensitivity: | 92% |
| Specificity: | 98% |
| Assay Type: | Qualitative |
Background
There is an urgent need to better understand COVID-19 antibody levels and duration of protective immunity for public health considerations. Coronavirus antibody testing in saliva has shown utility as a sensitive and specific serum alternative for large scale immune-surveillance studies (1). Importantly, IgG in saliva is derived from serum, so specificity and reactivity of salivary IgG directly reflects serum IgG reactivity, making saliva serology an attractive alternative (2, 3). Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is the RNA virus strain that causes COVID-19, the disease first identified in 2019. Initially designated 2019-nCoV (2019 novel coronavirus), COVID-19 was declared a public health emergency on January 30, 2020, and later declared a worldwide pandemic on March 11, 2020. SARS-CoV-2 spreads through human-to-human transmission and enters human cells through its binding of the angiotensin converting enzyme 2 (ACE2) receptor. Each virion consists of 4 structural proteins: S (spike protein), E (envelope), M (membrane), and N (nucleocapsid). For this assay, the N protein was chosen to maximize the likelihood of antibody detection, since it is the most immunodominant protein in the coronavirus family. Current research has shown that antibody levels decrease in the serum of COVID-19 patients over time (4) and vary depending on disease severity. In fact, a higher number of asymptomatic participants become seronegative at 60 days indicating a true decline over a 2-month period rather than an artifact of assay performance. Due to this, the current assay had antibody profiles benchmarked to a serum specific anti-N protein assay instead of PCR assay results.
References & Salivary SARS-CoV-2 Research
- Randad, PR., et al. (2021). COVID-19 serology at population scale: SARS-CoV-2-specific antibody responses in saliva. J Clin Microbiol.
- Hettegger, P., et al. (2019). High similarity of IgG antibody profiles in blood and saliva opens opportunities for saliva based serology. PloS one. 2019;14(6):e0218456.
- Heaney, JLJ., et al. (2018). The utility of saliva for the assessment of anti-pneumococcal antibodies: investigation of saliva as a marker of antibody status in serum Biomarkers. 23(2):115-22.
- Patel, M., et al. (2020). Change in Antibodies to SARS-CoV-2 Over 60 Days Among Health Care Personnel in Nashville, Tennessee. JAMA.
- Vabret N. (2020). Antibody responses to SARS-CoV-2 short-lived. Nat Rev Immunol. 20(9):519.
- Long QX., et al. (2020). Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 26(6):845-8.
 Contact: Salimetrics (USA)
Contact: Salimetrics (USA)