Need Help?
Ask an expert
1. How to collect Salivary Estriol
APPROVED SALIVARY ESTRIOL COLLECTION METHODS
Salivary Estriol Collection Protocol
Collection volume, general considerations, and basic guidelines to maximize salivary estriol sample integrity. Use this analyte-specific collection protocol to plan you collection methodology and sampling schemes.

2. How to Assay for Salivary Estriol
Send Saliva Samples to Salimetrics
Add to StudyEasy and accurate results from the most trusted Salivary Bioscience Laboratory.
Order Code5412
Diagnostic Salivary Estriol ELISA Kit (CE Mark)
Add to Study
Salimetrics Assay #1-2812
The Salimetrics Salivary Estriol (17b-triol) ELISA kit was designed for the laboratory determination of estriol in saliva samples. By making use of Salimetrics reagents, this saliva assay kit is ideal for low-level saliva estriol detection/measures with exceptional sensitivity. All Salimetrics salivary immunoassay kits are manufactured by experts in assay design and proven to deliver better results for saliva biomarkers. Read More...| Assay Protocol |
|---|
| Specifications | |
|---|---|
| Catalog#: | 1-2812 |
| Regulatory Status: | CE Mark |
| Format: | 96-well plate |
| Assay Time: | ~ 24 hrs |
| Sample Volume/Test: | 100 µL of X2 dilution |
| Sensitivity: | 1 pg/mL |
| Assay Range: | 5 pg/mL - 1215 pg/mL |
| Storage Requirements: | 2-8°C |
| Tests Per Kit | |
|---|---|
| Singlet: | 76 |
| Duplicate: | 38 |
| Target Analyte |
|---|
Technical Documentation
Salivary Estriol ELISA Kit
Add to Study
Salimetrics Assay #1-1802
The Salimetrics Salivary Estriol (17b-triol) ELISA kit was designed for the laboratory determination of estriol in saliva samples. By making use of Salimetrics reagents, this saliva assay kit is ideal for low-level saliva estriol detection/measures with exceptional sensitivity. All Salimetrics salivary immunoassay kits are manufactured by experts in assay design and proven to deliver better results for saliva biomarkers. Read More...| Assay Protocol |
|---|
| Rev. 02.21.25
|
| Specifications | |
|---|---|
| Catalog#: | 1-1802 |
| Regulatory Status: | RUO |
| Format: | 96-well plate |
| Assay Time: | ~ 3 hrs |
| Sample Volume/Test: | 25 µL |
| Sensitivity: | 16 pg/mL |
| Assay Range: | 20 pg/mL - 4860 pg/mL |
| Storage Requirements: | 2-8°C |
| Tests Per Kit | |
|---|---|
| Singlet: | 76 |
| Duplicate: | 38 |
| Target Analyte |
|---|
Technical Documentation
Assay Kit Overview
Intended Use
The Salimetrics Estriol/High Sensitivity (HS) Estriol Enzyme Immunoassay Kit is a competitive immunoassay specifically designed and validated for the quantitative measurement of salivary Estriol. It is not intended for diagnostic use. It is intended only for research use in humans and some animals. Salimetrics has not validated this kit for serum or plasma samples.
Introduction
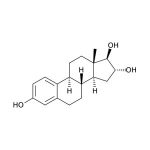
Estriol (1,3,5(10)-estratriene-3,16α,17β-triol; E3) is a female sex steroid hormone largely associated with pregnancy and fetal development. Fetal adrenal DHEA-S is metabolized in the fetal liver to 16-OH-DHEA-S, which is then converted to Estriol in the placenta. Near term, the fetus is the source of 90% of the 16-OH-DHEA-S in the normal human pregnancy. Maternal circulating Estriol levels rise progressively during pregnancy, reaching a peak in the third trimester. The physiological roles of Estriol in non-pregnant women are not well understood and are under investigation, particularly in connection with aging and post-menopausal health. With respect to estrogenic activity, Estriol is generally thought to be less potent than Estradiol or Estrone. However, with regard to nongenomic signaling pathways and functional responses in the pituitary, it has been pointed out that Estriol is a strong estrogen. In blood, the majority of Estriol is bound by serum proteins, with about 14-16% remaining unbound. Unbound Estriol enters saliva from blood via intracellular mechanisms, and correlation between serum and saliva samples is highly significant.
Salivary Estriol Assay Principle
This is a competitive immunoassay kit. Estriol in standards and samples compete with Estriol conjugated to horseradish peroxidase for the antibody binding sites on a microtitre plate. After incubation, unbound components are washed away. Bound Estriol Enzyme Conjugate is measured by the reaction of the horseradish peroxidase enzyme to the substrate tetramethylbenzidine (TMB). This reaction produces a blue color. A yellow color is formed after stopping the reaction with an acidic solution. The optical density is read on a standard plate reader at 450 nm. The amount of Estriol Enzyme Conjugate detected is inversely proportional to the amount of Estriol present in the sample.
3. Technical Summary
| Analyte Summary | |
|---|---|
| Analyte: | Estriol |
| Aliases: | 1,3,5(10)-estratriene-3,16α,17β-triol, E3 |
| Serum-Saliva Correlation: | 0.87 |
| *Optimum Collection Volume: | 175 μL |
| Assay Summary | |
|---|---|
| Methodology: | ELISA |
| Sensitivity: | 1 pg/mL |
| Standard Assay Range: | 20 pg/mL - 4860 pg/mL |
| HS Assay Range: | 5 pg/mL - 1215 pg/mL |
| Assay Type: | Quantitative |
Background
Estriol (1,3,5(10)-estratriene-3,16α,17β-triol; E3) is a female sex steroid hormone largely associated with pregnancy and fetal development. Fetal adrenal DHEA-S is metabolized in the fetal liver to 16α-hydroxy-DHEA-S, which is then converted to estriol in the placenta. (1,2) By the second trimester, about 90% of the estriol produced is derived from this fetal adrenal DHEA-S. (2) Maternal circulating estriol levels rise progressively during pregnancy, reaching a peak in the third trimester. Production of estriol depends on an intact maternal-placental-fetal unit, and maternal salivary estriol levels have been used to monitor fetal status during pregnancy. (2,3,4,5) Estriol is also used as part of the Tri- or Quad-Screen Test for detection of fetal genetic defects. (6,7) The physiological roles of estriol in non-pregnant women are not well understood and are under investigation. With respect to estrogenic activity, estriol is generally though to be less potent than estradiol or estrone. However, it has been pointed out that, with regard to nongenomic signaling pathways and functional responses in the pituitary, estriol is a strong estrogen. (8) Changes in levels of estriol and the other estrogens that occur due to menopause, pregnancy, and hormone replacement therapy have also been studied extensively for relationships to cancer susceptibility. (9-14) Estriol has also been investigated for its relation to autoimmune diseases such as multiple sclerosis and systemic lupus erythematosus, since pregnant women show significant reductions in symptoms during the last trimester of pregnancy. (15-19) Estriol has also been examined for its role in bone and lipid metabolism, and its function as a protective neurosteroid. (20,21) In blood the majority of estriol is bound by serum proteins, with about 14-16 % remaining unbound. (4,22) Unbound estriol enters saliva from blood via intracellular mechanisms, and salivary concentrations closely approximate unbound plasma concentrations. (3,23) There is virtually no protein-bound estriol in saliva. (4) Correlation between serum and saliva samples is highly significant. (24)
References & Salivary Estriol Research
-
-
- Troisi, R., Potischman, N., Roberts, J.M., et al. (2003). Maternal serum oestrogen and androgen concentrations in preeclamptic and uncomplicated pregnancies. Int J Epidemiol, 32(3), 455-60.
- Reis, F.M., D’Antona, D., Petraglia, F. (2002). Predictive value of hormone measurements in maternal and fetal complications of pregnancy. Endocr Rev, 23(2), 230-57.
- Vining, R.F., McGinley, R., Rice, B.V. (1983). Saliva estriol measurements: An alternative to the assay of serum unconjugated estriol in assessing feto-placental function. J Clin Endocrinol Metab, 56(3), 454-60.
- Kirkish, L.S., Compton, A.A., McCann, D.S. (1986). Salivary estriol as an index to fetal wellbeing. Clin Chem, 32(1 Pt 1), 71-75.
- Heine, R.P., McGregor, J.A., Goodwin, T.M., et al. (2000). Serial salivary estriol to detect an increased risk of preterm birth. Obstet Gynecol, 96(4), 490-97.
- Weintrob, N., Drouin, J., Vallette-Kasic, S., et al. (2005). Low estriol levels in the maternal triple-marker screen as a predictor of isolated adrenocorticotropic hormone deficiency caused by a new mutation in the TPIT gene. Pediatrics, 117(2), e322-7.
- Canick, J.A., MacRae, A.R. (2005). Second trimester serum markers. Semin Perinatol, 29(4), 203-8.
- Watson, C.S., Jeng, Y.-J., Kochukov, M.Y. (2008). Nongenomic actions of estradiol compared with estrone and estriol in pituitary tumor cell signaling and proliferation. FASEB J, 22(9), 3328-36.
- Jacobson, H.I., Lemanski, N., Agarwal, A., et al. (2010). A proposed unified mechanism for the reduction of human breast cancer risk by the hormones of pregnancy. Cancer Prev Res (Phil Pa), 3(2), 212-20.
- Larfors, G., Lambert, P.C., Lambe, M., et al. (2009). Placental weight and breast cancer survival in young women. Cancer Epid Biomark Prev, 18(3), 777-83.
-
- Hickey, M., Saunders, C., Partridge, A., et al. (2008). Practical clinical guidelines for assessing and managing menopausal symptoms after breast cancer. Ann Oncol, 19(10), 1669-80.
- Mascarenhas, C., Lambe, M., Bellocco, R., et al. (2006). Use of hormone replacement therapy before and after ovarian cancer diagnosis and ovarian cancer survival. Int J Cancer, 119(12), 2907-15.
- MacMahon, B. (2006). Epidemiology and the causes of breast cancer. Int J Cancer, 118(10), 2373-78.
- Lagiou, P., Samoli, E., Lagiou, A., et al. (2005). Maternal height, pregnancy estriol and birth weight in reference to breast cancer risk in Boston and Shanghai. Int J Cancer, 117(3), 494-98.
- Kassi, E., Moutsatsou, P. (2010). Estrogen receptor signaling and its relationship to cytokines in systemic lupus erythematosus. J Biomed Biotech, 2010:317452.
- Ding, J., Zhu, B.T. (2007). Unique effect of the pregnancy hormone estriol on antigen-induced production of specific antibodies in female BALB/c mice. Steroids, 73(3), 289-98.
- Rovenský, J. (2006). Rheumatic diseases and Klinefelter’s syndrome: Review. Scripta Med (Brno), 79(4), 237-46.
- Sicotte, N.L., Liva, S.M., Klutch, R., et al. (2002). Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann Neurol, 52(4), 421-28.
- Soldan, S.S., Alvarez-Retuerto, A.I., Sicotte, N.L., Voskuhl, R.R. (2003). Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. J Immunol, 171(11), 6267-74.
- Kika, G., Izumi, S.-I., Mori, A., et al. (2009). Beneficial aspect of oral estriol as hormone replacement therapy: Consideration on bone and lipid metabolism. Tokai J Exp Clin Med, 34(3), 92-98.
- Drača, S. (2006). Estriol and progesterone: A new role for sex hormones. Int J Biomed Sci, 2(4), 305-7.
- Moutsatsou, V., Oakey, R.E. (1988). Oestriol binding to plasma proteins. J Steroid Biochem, 29(3), 319-23.
- Vining, R.F., McGinley, R.A., Symons, R.G. (1983). Hormones in saliva: Mode of entry and consequent implications for clinical interpretation. Clin Chem, 29(10), 1752-56.
- Evans, J.J., Wilkinson, A.R., Aickin, D.R. (1984). Salivary estriol concentrations during normal pregnancies, and a comparison with plasma estriol. Clin Chem, 30(1), 120-21.
-
 Contact: Salimetrics (USA)
Contact: Salimetrics (USA)