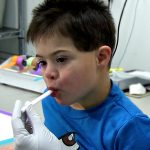
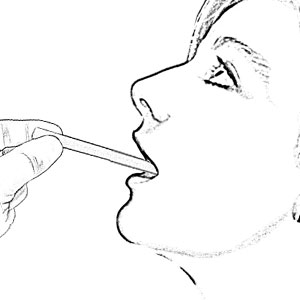
Saliva Collection for Children 6 Months - 6 Years Old
Experience convenient collection children from 6 months to 6 years of age (or from animals) with SalivaBio’s Children’s Swab (SCS) Method. The Children’s Swab method performs safely and effectively and features a 125mm swab, which is long enough to hold one end firmly while placing the other end in subject’s mouth (thus eliminating any choking hazard). The thin 8mm swab diameter suits smaller mouths, and the durable polymer withstands chewing. Each swab comes individually wrapped to minimize the possibility of environmental contaminants, causes no change in sample pH, and has verified recoveries. Read More...The volume of sample recovered is typically in the range of 200-1000 µL and is backed by Salimetrics QC, which verifies consistent lot-to-lot performance. Complete with SalivaBio Children’s Swab (SCS), swab storage tube, and sample collection instructions, this saliva collection system is ready to use in the field or on-site. If your analyte is not listed below, a pilot study may reveal a high correlation in your subject group, which will enable you to utilize this method effectively.
Technical Summary
| Method Instructions |
|---|
| Specifications | |
|---|---|
| Intended Use: | Children 6 months- 6 years old |
| Volume Capacity: | 2 mL |
| Regulatory Status: | FDA Listed, CE Marked |
| Material Composition: | Synthetic Swab |
| Swab Size: | 8 mm x 125 mm |
| Individually Wrapped: | Yes |
| What's Included | |
|---|---|
| SalivaBio Children's Swab Device (5001.06) | 50 pcs |
| Swab Storage Tube (5001.05) | 50 pcs |
| Approved for Analytes |
|---|
| Cortisol, Alpha-Amylase, Cotinine, IgG/IgM, Interleukin-1 Beta, Secretory Immunoglobulin A, Testosterone, DNA Analysis, Uric Acid |
| Technical Documentation |
|---|
| Saliva Collection Handbook
Rigor and Reproducibility: How Good Are Your Saliva Samples? |
How To Collect Saliva: The SalivaBio Children's Swab
Recommended Handling & Storage Accessories
4″ Swab/Conical Tube Storage Box
Moisture-resistant laminated fiberboard boxes are ideal for freezer and refrigerator storage. The 7 x 7 grid insert makes easy work of sample organization.
| Specifications | |
|---|---|
|
Item# |
5023.00 |
| Size: | 5.5″ x 5.5″ x 4″ |
| Qty: | Each |
Bar-Coded Sample Labels
Small polypropylene labels which adhere to tubes frozen to -80°C and will not fall off upon thawing. Sample ID number, study name and barcode are pre-printed onto the 1-in square labels ensuring positive sample identification during saliva collections.
| Specifications | |
|---|---|
|
Item# |
5009.07 |
| Size: | 26 mm x 26 mm (1″ x 1″) |
| Qty: | Each |

How Can we help?
 Contact: Salimetrics (USA)
Contact: Salimetrics (USA)
Test Your DBS samples with Salimetrics!
Experts in Saliva & Dried Blood Spot Testing for Research
The best science for your study
OR CALL 800.790.2258
X