Drop Date: August 2021
Infectious Diseases – Salivary Antibody Assays Track Exposure and Infection
In This Drop: Infectious Diseases – Salivary Antibody Assays Track Exposure and Infection
Although the state of the pandemic still seems to fluctuate from one day to the next, one thing remains clear: COVID-19 has shown us the benefits of saliva as a sample type are unprecedented for infectious disease research.
Now, investigators are needed to build on this foundation and further define how saliva, as a serum alternative in epidemiological seroprevalence and surveillance studies, is beneficial for large-scale, population-based studies. Saliva sampling is easy to access through central collection sites and home collection, enabling high-quality samples with better participant compliance and facilitating increased testing capacity – all at critical economies of scale. In this bulletin, we have summarized the current state of the industry, how you can apply the latest tools to support new, innovative research programs, and how our team is here to assist in the next chapter of discovery.
Antibody Isotypes in Saliva
Saliva and other oral fluid collections contain readily measurable pathogen-specific antibodies whose reactivities mirror that found in the blood. These include antibodies of the Immunoglobulin A (IgA), Immunoglobulin G (IgG), and Immunoglobulin M (IgM) isotype. Secretory IgA is assembled into dimers associated with an additional protein (the J chain) when secreted into mucosal fluids (including saliva), while the oral source of IgG and IgM is from serum diffusing into saliva through the capillary beds of the gingival space between the gums and teeth. Enriched oral fluids such as gingival crevicular fluid (GCF) or oral mucosal transudate (OMT) have even higher concentrations of both IgG and IgM. Importantly, these antibody-rich samples can be self-collected efficiently with the proper collection methods and protocols. This enables efficient surveying of pathogen-specific antibodies in saliva, which will allow for tracking pathogen or vaccine exposure with differentiation between recent (IgM) or historical (IgG) exposure.
For an in-depth review of studies involving salivary pathogen-specific antibody makers, see table 13.1, in Chapter 13 of Salivary Bioscience: Foundations (1).
Saliva in Population-Based Infectious Disease Epidemiology and Surveillance
Evidence is building that shows saliva can facilitate population-level seroprevalence. Pairing this information with identifying risk factors for exposure and transmission, researchers can lead effective strategies for preventing and monitoring disease spread and containment.
A 2017 publication from Augustine et al. (2), explored the utility of saliva for epidemiological studies by investigating if visitors at Boquerón Beach in Puerto Rico were exposed to waterborne pathogens. Due to the ease of collection, visitors were able to collect saliva on-site. Results of the study indicated that more than “two-thirds of beachgoers were previously infected by at least one of the included waterborne pathogens” (2). Knowing this information can help determine exposure to specific detectable antibodies over a specific community or population.
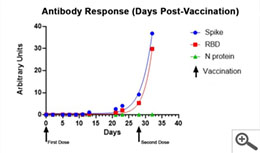
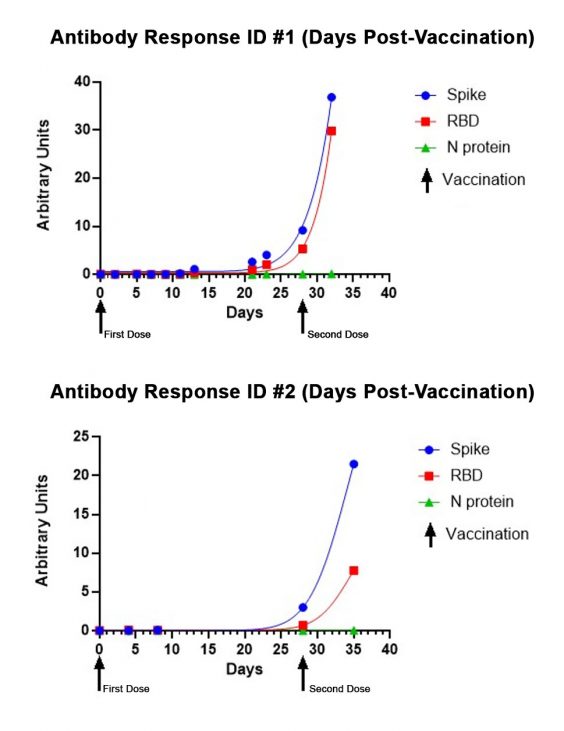
In 2021, Salimetrics released the SARS-CoV-2 IgG Antibody Assay Panel 3-Plex (S-Protein, N-Protein, & RBD), featuring a 98.8% specificity and a 96.5% specificity, as part of their laboratory testing offering. This assay was specifically validated for use without specialized collection devices. The SARS-CoV-2 IgG Antibody Panel can also effectively track the antibody response to the vaccine or a natural infection over time and provides relative levels by including a reference standard. Below are representative performance data from individuals starting on the first day of immunization with the Moderna vaccine through a week after receiving their second dose of the vaccine. The lack of N-protein reactivity (shown in green) distinguishes the SARS-CoV-2 vaccination since the vaccine does not encode N-protein. In the graph below, you can see a moderate increase in Spike/RBD reactivity up to the second immunization (indicated by arrow) and the exponential increase in reactivity post-second immunization.
Saliva can also be used to monitor seroconversion, not just seroprevalence. For example, with COVID-19, investigators can observe patients post-infection to determine if they begin making antibodies to SARS-CoV-2, and which antigen specific antibodies are made – which is a factor in determining disease prognosis (3).
Use of Saliva in the Surveillance of Vaccine Preventable Diseases (VPDs)
Most notably, saliva can be used to inform disease control strategies and guide immunization programs. When a disease presents itself at varying levels of intensity, such as observed with COVID-19, relying on the presentation of clinical symptoms is insufficient to monitor the spread of disease exposure. In this case, saliva represents an unprecedented ability to evaluate large-scale surveys of historical exposure through pathogen-specific IgG. At home, self-collection and the use of drop-off or mail-in sample returns to a central testing site differentiates saliva from serum or plasma by minimizing exposure to health care workers at collection sites and enabling a geographical scale of magnitude that is difficult to achieve with traditional central collection sites. These benefits are realized, for instance, when monitoring vaccination campaigns and routine immunizations or seroprevalence during outbreaks.
For example, Morris Cunnington completed a national study for hepatitis A virus (HAV) surveillance using oral fluid as a sample type (4). The findings showed that self-collection of oral fluids for serology was a practical way of sampling, and HAV-specific IgG can be used to accurately evaluate the exposure of a population when compared to blood. In this publication, applying specific demographic and social data measurements along with the antibody data provided strong epidemiological associations.
Another example of the utility of saliva for surveillance was to track the progression of Hepatitis A, B, and C. In a 2007 publication, a cross-sectional study investigated the prevalence of hepatitis compared to previous studies. “The 2003 findings of the cross-sectional oral fluid IgG survey in Belgium, was compared to a previous study 10 years earlier and indicated that Hep A and C may have been less of a burden than estimated in 1993” (5).
The staggering benefits of saliva can also be noted from a study that analyzed rubella cases in 2010. “For rubella, the incidence of rubella cases increased from 0.5 to 0.77 per 1,000,000 population when oral fluid testing was added” (6). In this study, oral fluid provided researchers with more specific information for those who had confirmed rubella cases, and allowed researchers to form strategies that would be effective for eliminating rubella in the UK based on their results.
In all cases, saliva can be collected safely in population-based settings without exposure risk and with less chance of participant error using self-collected specimens. Utilizing this methodology, investigators can operate on a large scale with increased participant compliance over blood-based sampling.
Utility of Saliva in Diagnostic Testing
The utility of saliva as a diagnostic specimen is continuously evolving. As antibodies in saliva originate in blood, they are bioidentical to their serum counterparts and therefore, can be utilized in a diagnostic capacity. Most current salivary testing under this umbrella is captured as a method to screen high-risk participants or monitor active treatments and therapies. While epidemiological surveys generally do not require FDA approval, saliva will play a unique role in future diagnostics as the accuracy of saliva continues to improve with new collection techniques and a broadening understanding of saliva’s biological capacity. This advancement will soon lead to the development of precision Point of Care devices and saliva-based companion diagnostics.
Generally, in diagnostic applications, one limiting factor in successful testing is how the sample is collected. Due to the heterogeneous nature of saliva, particular attention must be placed on collecting the right sample for analysis. For antibody testing, this may require collecting an antibody enriched sample such as Gingival Crevicular Fluid (GCF) or Oral Mucosal Transudate (OMT). Salimetrics, in collaboration with SalivaBio, has focused on addressing sample collection concerns to enable a new wave of possibilities by facilitating better results through qualified and validated collection methods. Through this initiative, new methods for specific sample collection applications are on the horizon.
Empowering Solutions from Salimetrics
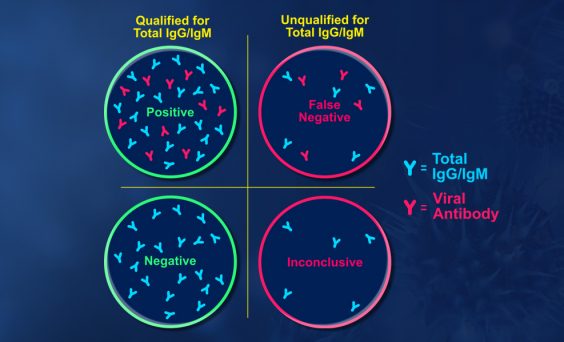
Salimetrics has developed and optimized tools required for use in epidemiological research and surveillance and has validated these tools to provide researchers a ready-to-use platform for infectious disease research. With the recent introduction of the Salimetrics Salivary Total Human IgG ELISA Kit and the Salivary Total Human IgM ELISA Kit, a new suite of tools is now available for researchers. Combined with Salimetrics Salivary Human SIgA ELISA Kit, this line of infectious disease research tools has been optimized to improve the specificity and sensitivity of corresponding pathogen-specific serological assays. Specifically, these assay tools minimize false negative determinations in pathogen-specific serological assays by excluding samples that have insufficient total antibody levels. In the current state of the pandemic, researchers can take advantage of this opportunity to understand the impact of COVID-19 on a biological level. Researchers can more fully understand the impact of COVID-19 in their study population with Salimetrics SARS-CoV-2 (IgG) Antibody Assay, 3-Plex (S-Protein, N-Protein, & RBD) – or with the Salivary SARS-CoV-2, N Antibody ELISA Kit.
If you’re exploring the next generation of infectious disease research with saliva or capturing the impact of COVID-19 stress-related exposure, Salimetrics is here to support your research and help you find solutions to your research challenges. For more in-depth information, the full scope of antibodies in saliva for infectious disease research can be found in Salivary Bioscience: Foundations, Chapter 13: The Utility of Antibodies in Saliva to Measure Pathogen Exposure and Infection.
REFERENCES & RELATED RESEARCH
- Randad P.R. et al. (2020) The Utility of Antibodies in Saliva to Measure Pathogen Exposure and Infection. In: Granger D., Taylor M. (eds) Salivary Bioscience. Springer, Cham. https://www.springer.com/gp/book/9783030357832
- Augustine, et al., Immunoprevalence to Six Waterborne Pathogens in Beachgoers at Boquerón Beach, Puerto Rico: Application of a Microsphere-Based Salivary Antibody Multiplex Immunoassay. Frontiers in Public Health. 2017; 1;5:84. https://www.frontiersin.org/articles/10.3389/fpubh.2017.00084/full
- Randad, et al., COVID-19 serology at population scale: SARS-CoV-2-specific antibody responses in saliva. medRxiv. 2020; 2020.05.24.20112300. https://pubmed.ncbi.nlm.nih.gov/32511537/
- Morris-Cunnington, M. C., et al., A Population- based Seroprevalence Study of Hepatitis A Virus Using Oral Fluid in England and Wales. American Journal of Epidemiology, 2004; 159(8), 786–794. https://pubmed.ncbi.nlm.nih.gov/15051588/
- Quoilin, et al., A Population-Based Prevalence Study of Hepatitis A, B and C Virus Using Oral Fluid in Flanders, Belgium. European Journal of Epidemiology, 2007; 22(3), 195–202. https://pubmed.ncbi.nlm.nih.gov/17356926/
- Manikkavasagan, G.et al., (2010). Oral Fluid Testing during 10 Years of Rubella Elimination, England and Wales. Emerging Infectious Diseases, 2010; 16(10), 1532–1538.
https://wwwnc.cdc.gov/eid/article/16/10/10-0560_article
Additional resources not directly referenced:
- Campbell, H. et al., Oral fluid Testing for Pertussis, England and Wales, June 2007–August 2009. Emerging Infectious Diseases, (2014). ; 20(6), 968–975. https://wwwnc.cdc.gov/eid/article/20/6/13-1069_article
- Mortimer, P. P., & Parry, J. V. (1991). Non-invasive Virological Diagnosis: Are saliva and Urine Specimens Adequate Substitutes for Blood? Reviews in Medical Virology, 1991; https://onlinelibrary.wiley.com/doi/10.1002/rmv.1980010204
- Ramsay, M. E.et al., The Elimination of Indigenous Measles Transmission in England and Wales. The Journal of Infectious Diseases, 2003; 187 1233(s1), S198–S207. https://pubmed.ncbi.nlm.nih.gov/12721914/
*Note: Salimetrics provides this information for research use only (RUO). Information is not provided to promote off-label use of medical devices. Please consult the full-text article.
 Contact: Salimetrics (USA)
Contact: Salimetrics (USA)