Salimetrics Assay #1-2602
Salimetrics salivary assay kits are expertly designed, developed and validated to ensure accuracy in saliva and proven to deliver precision results for saliva biomarkers.The Salimetrics Salivary 17 Alpha-OHP Enzyme Immunoassay Kit is specifically designed to standardize the detection of 17 Alpha-OHP in saliva samples for research and biomedical laboratories. To ensure the most accurate results, this salivary immunoassay is designed using a matrix that matches saliva. The level of 17 Alpha-OHP in saliva (pg/mL) is significantly lower than levels in blood (ng/mL). The standard curve range is sensitive enough to capture individual differences in the 17 Alpha-OHP levels expected in saliva. The current protocol uses only 50 μL of saliva per test. No separation or extractions are necessary. This 17 Alpha-OHP assay kit has also been formatted to minimize cross reactivity for related steroids.Technical Summary
| Assay Protocol |
|---|
| Rev. 04.19.19
|
| Specifications | |
|---|---|
| Catalog#: | 1-2602 |
| Regulatory Status: | RUO |
| Format: | 96-well plate |
| Assay Time: | ~ 3 hrs |
| Sample Volume/Test: | 50 µL |
| Sensitivity: | 3 pg/mL |
| Assay Range: | 5.1 pg/mL - 500pg/mL |
| Storage Requirements: | 2-8°C |
| Tests Per Kit | |
|---|---|
| Singlet: | 76 |
| Duplicate: | 38 |
| Target Analyte |
|---|
| Technical Documentation |
|---|
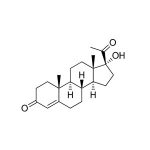
Salivary 17 Alpha-Hydroxyprogesterone Assay Kit Overview
Intended Use
The Salimetrics 17 α-Hydroxyprogesterone (17-OH Progesterone) Enzyme Immunoassay Kit is a competitive immunoassay specifically designed and validated for the quantitative measurement of salivary 17-OH Progesterone. It is not intended for diagnostic use. It is intended only for research use in humans and some animals. Salimetrics has not validated this kit for serum or plasma samples.
Introduction
17-OH Progesterone (17-hydroxy-4-pregnene-3,20-dione) is a steroid hormone produced in the adrenal glands and the gonads. Immediate precursors are progesterone or 17- hydroxypregnenolone. In the human adrenal gland, 17-OH Progesterone is metabolized to 11- deoxycortisol, which is then further converted to the main end product cortisol. Production and conversion of 17-OH Progesterone are therefore essential to normal adrenal function. 17-OH Progesterone also serves as a precursor to the androgens and the estrogens, with androstenedione serving as the intermediary metabolite. The enzymatic activity that converts 17-OH Progesterone into androstenedione varies considerably according to species, however in the human adrenal gland, testis, and ovary, 17-OH Progesterone conversion to androstenedione is inefficient, and DHEA instead serves as the main precursor to androstenedione. 17-OH Progesterone produced in the human gonadal tissues is therefore largely an end product that enters the circulation, and, in the absence of adrenal dysfunction, circulating 17-OH Progesterone levels reflect gonadal rather than adrenal production. 17-OH Progesterone levels correlate with the changes in circulating estrogen levels seen during the follicular and luteal phases of the menstrual cycle in women. 17-OH Progesterone levels follow a circadian rhythm similar to that of cortisol, with highest values in the morning and a nadir in the evening. 17-OH Progesterone levels in humans are high at birth and drop rapidly over the first few days of life; they continue to decline more slowly over the first year. Levels remain low until they rise again during puberty. To ensure the most accurate results, this salivary immunoassay is designed using a matrix that matches saliva. The level of 17-OH Progesterone in saliva (pg/mL) is significantly lower than levels in the general circulation (ng/mL). The standard curve range is sensitive enough to capture individual differences in the 17-OH Progesterone levels expected in saliva. The current protocol uses only 50 µL of saliva per test. No separation or extractions are necessary.
17-OH Progesterone Assay Principle
This is a competitive immunoassay kit. 17-OH Progesterone in standards and samples compete with 17-OH Progesterone conjugated to horseradish peroxidase for the antibody binding sites on a microtitre plate. After incubation, unbound components are washed away. Bound 17-OH Progesterone Enzyme Conjugate is measured by the reaction of the horseradish peroxidase enzyme to the substrate tetramethylbenzidine (TMB). This reaction produces a blue color. A yellow color is formed after stopping the reaction with an acidic solution. The optical density is read on a standard plate reader at 450 nm. The amount of 17-OH Progesterone Enzyme Conjugate detected is inversely proportional to the amount of 17-OH Progesterone present in the sample.
References & Salivary 17-Alpha OHP Research
-
- Pasquali, R., Patton, L., Pocognoli, P., et al. (2007). 17-Hydroxyprogesterone responses to gonadotropin-releasing hormone disclose distinct phenotypes of functional ovarian hyperandrogenism and polycystic ovary syndrome. J Clin Endocrin Metab, 92(11), 4208-17.
- Şahin, Y., Keleştimur, F. (1997). 17-Hydroxyprogesterone responses to gonadotrophin-releasing hormone agonist buserelin and adrenocorticotrophin in polycystic ovary syndrome: Investigation of adrenal and ovarian cytochrome P450c17α dysregulation. Hum Reprod, 12(5), 910-13.
- Strott, C.A., Yoshimi, T., Lipsett, M.B. (1969). Plasma progesterone and 17-hydroxyprogesterone in normal men and children with congenital adrenal hyperplasia. J Clin Invest, 48(5), 930-39.
- Weliky, I., Engel, L.L. (1962). Metabolism of progesterone-4-C14 and pregnenolone-7α-H3 by human adrenal tissue. J Biol Chem, 238(4), 1302-7.
- Santos, C.M., Abad, L.R., Cua, S.C. & Domingo, C.F. (2003). Monitoring congenital adrenal hyperplasia using blood spot 17-hydroxyprogesterone assay. Southeast Asian J Trop Med Public Health, 34(Suppl 3), 174-8.
- Sack, J., Front, H., Kaiserman, I., Schreiber, M. (1997). 21-hydroxylase deficiency: Screening and incidence in Israel. Horm Res, 48(3), 115-9.
- New, M.I., Levine, L.S. (1984). Congenital adrenal hyperplasia. Monographs on Endocrinology, 26, 1-88.
- Van der Kamp, H.J, Wit, J.M. (2004). Neonatal screening for congenital adrenal hyperplasia. Eur J Endocrinol, 151(Suppl. 3), U71-5.
- Hughes, I.A., Winter, J.S. (1976). The application of a serum 17-OH progesterone radioimmunoassay to the diagnosis and management of congenital adrenal hyperplasia. J Pediatrics, 88(5), 766-73.
- Gröschl, M., Rauh, M., Dörr, H.-G. (2003). Circadian rhythm of salivary cortisol, 17α-hydroxyprogesterone, and progesterone in healthy children. Clin Chem, 49(10), 1688-91.
-
- Goudas, V.T., Dumesic, D.A. (1997). Polycystic ovary syndrome. Endocrinol Metab Clin North Am, 26(4), 893-912.
- Vining, R.F., McGinley, R.A. (1987). The measurement of hormones in saliva: Possibilities and pitfalls. J Ster Biochem, 27(1-3), 81-94.
 Contact: Salimetrics (USA)
Contact: Salimetrics (USA)