Need Help?
Ask an expert
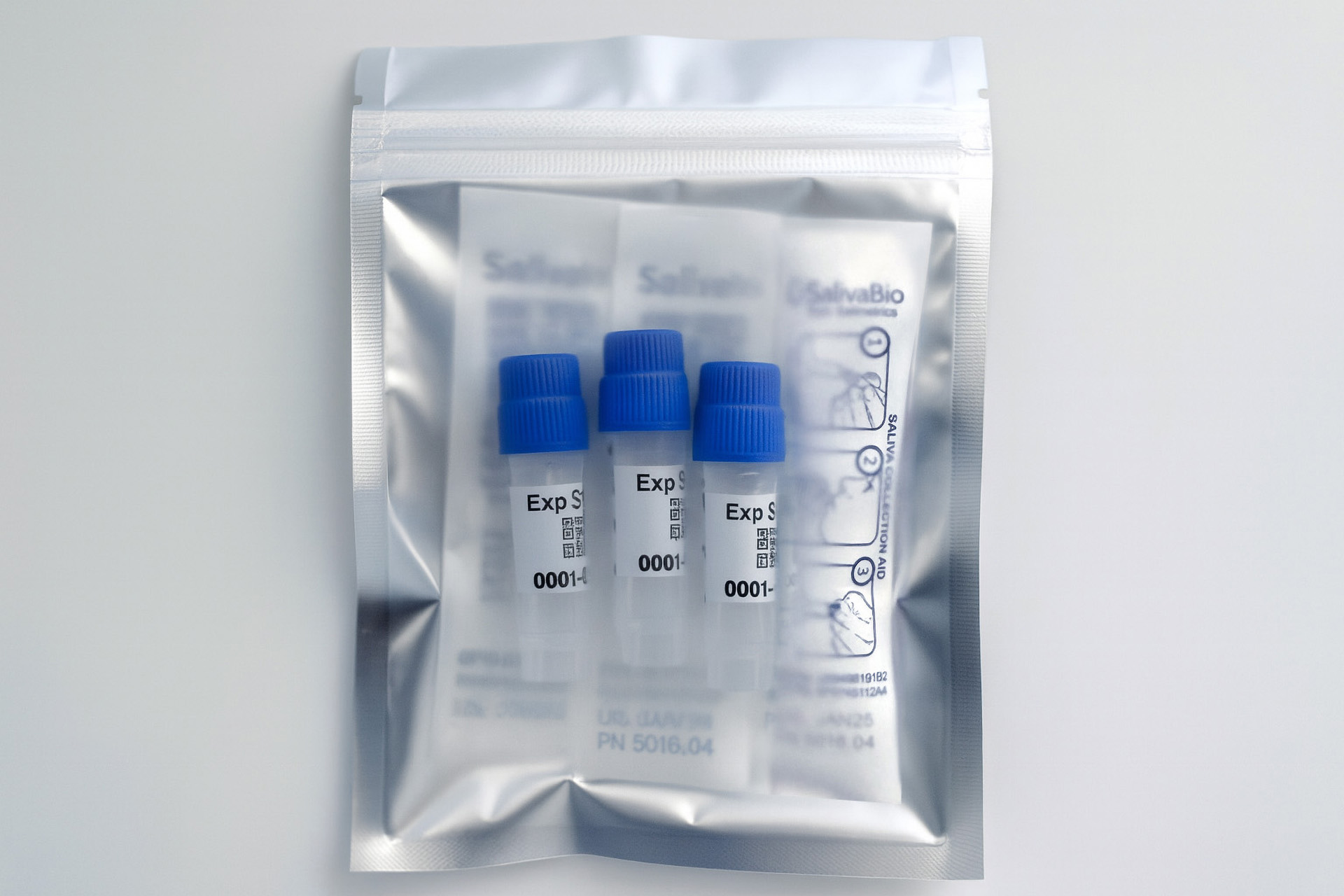
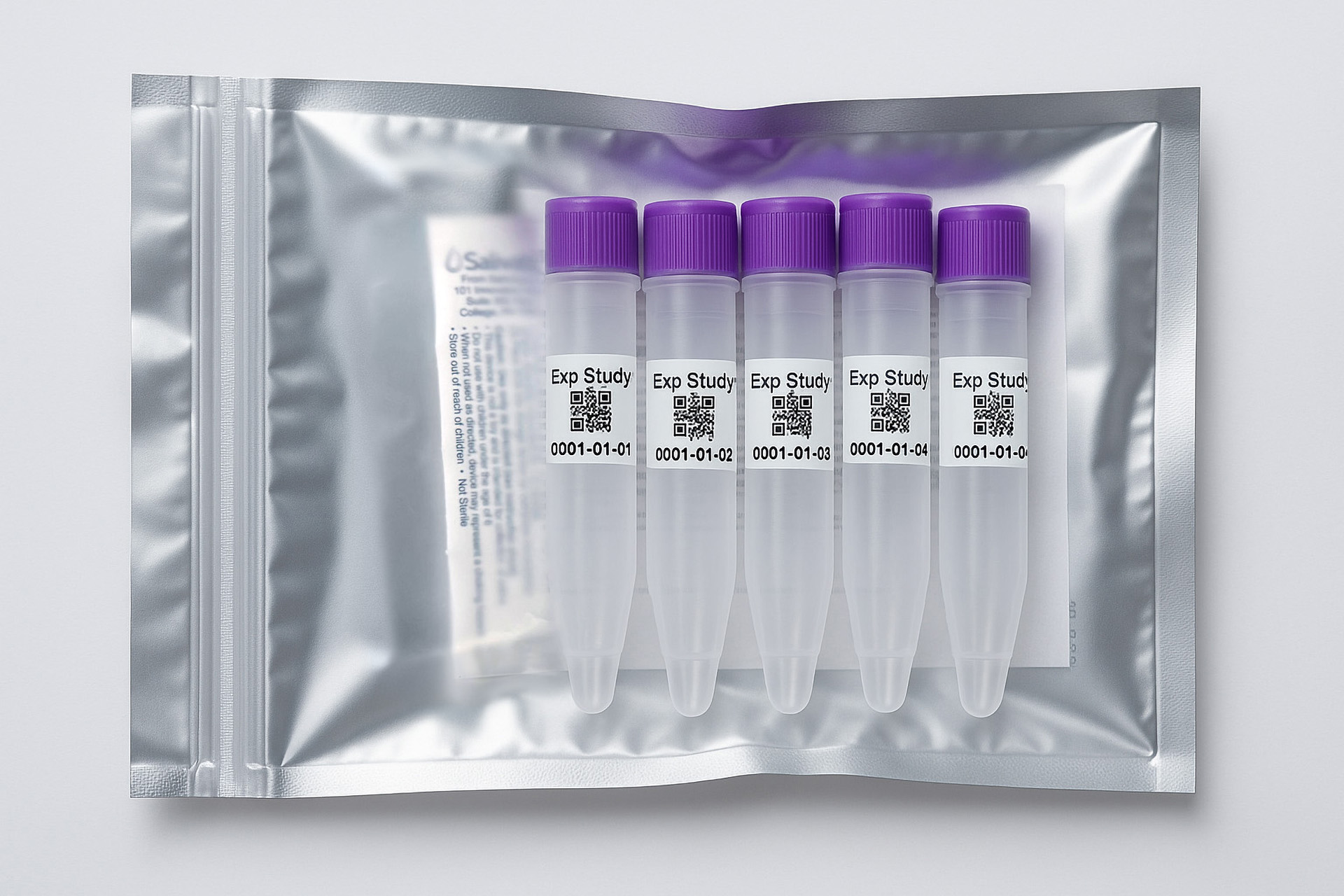
1. How to collect Salivary Testosterone
APPROVED SALIVARY TESTOSTERONE COLLECTION METHODS
Salivary Testosterone Collection Protocol
Collection volume, general considerations, and basic guidelines to maximize salivary testosterone sample integrity. Use this analyte-specific collection protocol to plan you collection methodology and sampling schemes.

2. How to Assay for Salivary Testosterone
Send Saliva Samples to Salimetrics
Add to StudyEasy and accurate results from the Salimetrics CoreLab+ Laboratory.
Order Code5138
Salivary Testosterone ELISA Kit
Add to Study
Salimetrics Assay #1-2402
The Salimetrics Salivary Testosterone Enzyme Immunoassay Kit was specifically designed to standardize the detection of testosterone in saliva samples for research and biomedical laboratories. Using a small sample volume, this assay kit has an extended range that spans the expected testosterone levels found in human saliva. The average inter- and intra-assay precision coefficients of variation are low with no deleterious matrix effects often found in saliva which are characterized through dilution- and spike-recovery validation procedures. This testosterone assay kit has also been formatted to minimize cross reactivity for related steroids. Testosterone exhibits a diurnal rhythm, with highest levels in the morning and a nadir around midnight. In men, testosterone plays an important role in the development of male reproductive tissues including the testes and prostate, as well as promoting secondary sexual characteristics such as increased muscle, bone mass, and hair growth. In blood, only 1-10% of testosterone is in its unbound or biologically active form. The remaining testosterone is bound to serum proteins whereas the majority of testosterone in saliva is not protein-bound. Salivary testosterone levels are unaffected by salivary flow rate. The serum-saliva correlation for testosterone is very high for males, but only modest for females. Read More...| Assay Protocol |
|---|
| Rev. 08.15.25
|
| Specifications | |
|---|---|
| Catalog#: | 1-2402 |
| Regulatory Status: | RUO |
| Format: | 96-well plate |
| Assay Time: | ~ 2 hrs |
| Sample Volume/Test: | 25 µL |
| Sensitivity: | 1 pg/mL |
| Assay Range: | 6.1 pg/mL - 600 pg/mL |
| Storage Requirements: | 2-8°C |
| Tests Per Kit | |
|---|---|
| Singlet: | 78 |
| Duplicate: | 39 |
| Target Analyte |
|---|
Technical Documentation
Assay Kit Overview
Intended Use
The Salimetrics Testosterone Enzyme Immunoassay Kit is a competitive immunoassay specifically designed and validated for the quantitative measurement of salivary Testosterone. It is not intended for diagnostic use. It is intended only for research use in humans and some animals. Salimetrics has not validated this kit for serum or plasma samples.
Introduction
Testosterone is an anabolic steroid hormone synthesized from androstenedione in the Leydig cells of the testes of males and, in smaller quantities, in the ovaries of females. Small amounts are also secreted by the adrenal glands in both sexes. Testosterone production also occurs in peripheral tissues by conversion of circulating DHEA-S, DHEA, and androstenedione. Testosterone exhibits a diurnal rhythm, with highest levels in the morning and a nadir around midnight. In men, Testosterone plays an important role in the development of male reproductive tissues including the testes and prostate, as well as promoting secondary sexual characteristics such as increased muscle, bone mass, and hair growth. In blood, only 1-10% of Testosterone is in its unbound or biologically active form. The remaining Testosterone is bound to serum proteins. Unbound Testosterone enters saliva via intracellular mechanisms, and in saliva the majority of Testosterone is not protein-bound. Salivary Testosterone levels are unaffected by salivary flow rate. The serum-saliva correlation for Testosterone is very high for males, but only modest for females, possibly because women’s values often fall near the bottom of the measurable range for both serum and saliva immunoassay kits.
Salivary Testosterone Assay Principle
This is a competitive immunoassay kit. Testosterone in standards and samples compete with Testosterone conjugated to horseradish peroxidase for the antibody binding sites on a microtitre plate. After incubation, unbound components are washed away. Bound Testosterone Enzyme Conjugate is measured by the reaction of the horseradish peroxidase enzyme to the substrate tetramethylbenzidine (TMB). This reaction produces a blue color. A yellow color is formed after stopping the reaction with an acidic solution. The optical density is read on a standard plate reader at 450 nm. The amount of Testosterone Enzyme Conjugate detected is inversely proportional to the amount of Testosterone present in the sample.
Diagnostic Testosterone ELISA Kit (CE Mark)
Add to Study
Salimetrics Assay #1-2312 (in vitro diagnostic use)
The Salimetrics Salivary Testosterone Enzyme Immunoassay Kit was specifically designed to standardize the detection of testosterone in saliva samples for research and biomedical laboratories. Using a small sample volume, this assay kit has an extended range that spans the expected testosterone levels found in human saliva. The average inter- and intra-assay precision coefficients of variation are low with no deleterious matrix effects often found in saliva which are characterized through dilution- and spike-recovery validation procedures. This testosterone assay kit has also been formatted to minimize cross reactivity for related steroids. Read More...| Assay Protocol |
|---|
| Specifications | |
|---|---|
| Catalog#: | 1-2312 |
| Regulatory Status: | CE Mark |
| Format: | 96-well plate |
| Assay Time: | ~2 hrs |
| Sample Volume/Test: | 25 µL |
| Sensitivity: | 1 pg/mL |
| Assay Range: | 6.1 pg/mL - 600 pg/mL |
| Storage Requirements: | 2-8°C |
| Tests Per Kit | |
|---|---|
| Singlet: | 78 |
| Duplicate: | 39 |
| Target Analyte |
|---|
Technical Documentation
3. Technical Summary
| Analyte Summary: | |
|---|---|
| Analyte: | Testosterone |
| Aliases: | Androst-4-en-17β-ol-3-one |
| Serum-Saliva Correlation: | 0.96 |
| *Optimum Collection Volume: | 75 μL |
| Assay Summary: | |
|---|---|
| Methodology: | ELISA |
| Sensitivity: | 1 pg/mL |
| Assay Range: | 6.1 pg/mL - 600 pg/mL |
| Assay Type: | Quantitative |
Background
Testosterone is an anabolic steroid hormone synthesized from androstenedione in the Leydig cells of the testes of males and, in smaller quantities, in the ovaries of females. (1) Small amounts are also secreted by the adrenal glands in both sexes. In both men and women a large portion of total testosterone production occurs in peripheral tissues by conversion of circulating DHEA-S, DHEA, and androstenedione. In post-menopausal women, the ovaries and peripheral tissues continue to produce testosterone and other androgens, which then serve as precursors for the synthesis of estradiol in the peripheral tissues. The conversion of precursors into testosterone and the estrogens in peripheral tissues allows these steroids to be delivered to the appropriate tissues without leakage of significant amounts into the circulation, avoiding undesirable effects of high circulating levels. (2-4) Testosterone exhibits a diurnal rhythm, with highest levels in the morning and a nadir around midnight. (5,6) In men, testosterone plays an important role in the development of male reproductive tissues including the testis and prostate, as well as promoting secondary sexual characteristics such as increased muscle, bone mass, and hair growth. (7,8) Additionally, testosterone is essential for health and well-being, stamina, sexual function, cardiovascular health, and immune protection. (9-12) Testosterone measurements are typically used for clinical evaluation of hypogonadism in males and hyperandrogenic states in females. (13-15) In blood, only 1 to 15% of testosterone is in its unbound or biologically active form. The remaining testosterone is bound to serum proteins. Unbound testosterone enters the saliva via intracellular mechanisms, and in saliva the majority of testosterone is not protein-bound. (16) The serum-saliva correlation for testosterone is very high for males, but only modest for females, possibly because women’s values often fall near the bottom of the measurable range for both serum and saliva immunoassay kits. (17,18)
References & Salivary Testosterone Research
-
- Labrie, F., Luu-The, V., Bélanger, A., et al. (2005). Is dehydroepiandrosterone a hormone? J Endocrinol, 187(2), 169-96.
- Nakamura, Y., Hornsby, P.J., Casson, P., et al. (2008). Type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. J Clin Endocrinol Metab, 94(6), 2192-98.
- Burger, H.G. (2002). Androgen production in women. Fertil Steril, 77(Suppl 4), S3-5.
- Labrie, F., Bélanger, A., Cusan, L., Candas, B. (1997). Physiological changes in dehydroepiandrosterone are not reflected by serum levels of active androgens and estrogens but of their metabolites: Intracrinology. J Clin Endocrinol Metab, 82(8), 2403-9.
- Ankarberg, C., Norjavaara, E. (1999). Diurnal rhythm of testosterone secretion before and throughout puberty in healthy girls: Correlation with 17β-estradiol and dehydroepiandrosterone sulfate. J Clin Endocrinol Metab, 84(3), 975-84.
- Diver, M.J., Imtiaz, K.E., Ahmad, A.M., et al. (2003). Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clin Endocrinol (Oxf), 58(6), 710-17.
- Rogol, A.D., Clark, P.A., Roemmich, J.N. (2000). Growth and pubertal development in children and adolescents: Effects of diet and physical activity. Am J Clin Nutr, 72(2 Suppl.), 521S-28.
- Snyder, P.J., Peachey, H., Berlin, J.A., et al. (2000). Effects of testosterone replacement in hypogonadal men. J Clin Endocinol Metab, 85(8), 2670-77.
- Tibblin, G., Adlerberth, A., Lindstedt, G., Björntorp, P. (1996). The pituitary-gonadal axis and health in elderly men: A study of men born in 1913. Diabetes, 45(11), 1605-9.
- Davis, S.R., Tran, J. (2001). Testosterone influences libido and well being in women. Trends Endocrinol Metab, 12(1), 33-7.
-
- Wang, C., Alexander, G., Berman, N., et al. (1996). Testosterone replacement therapy improves mood in hypogonadal men: A clinical research center study. J Clin Endocrinol Metab, 81(10), 3578-83.
- Malkin, C.J., Pugh, P.J., West, J.N., et al. (2006). Testosterone therapy in men with moderate severity heart failure: A double-blind randomized placebo controlled trial. Eur Heart J, 27(1), 57-64.
- Bhasin, S., Bremner, W.J. (1997). Clinical review 85: Emerging issues in androgen replacement therapy. J Clin Endocrinol Metab, 82(1), 3-8.
- Gibson, M., Lackritz, R., Schiff, I., Tulchinsky, D. (1980). Abnormal adrenal responses to adrenocorticotropic hormone in hyperandrogenic women. Fertil Steril, 33(1), 43-8.
- Rodin, A., Thakkar, H., Taylor, N., Clayton, R. (1994). Hyperandrogenism in polycystic ovary syndrome: Evidence of dysregulation of 11β-hydroxysteroid dehydrogenase. N Eng J Med, 330(7), 460-65.
- Vining, R.F., MicGinley, R.A. (1987). The measurement of hormones in saliva: Possibilities and pitfalls. J Steroid Biochem, 27(1-3), 81-94.
- Wang, C., Plymate, S., Nieschlag, E., Paulsen, C.A. (1981). Salivary testosterone in men: Further evidence of a direct correlation with free serum testosterone. J Clin Endocrinol Metab, 53(5), 1021-24.
- Rollin, G. (2010). The trials of testosterone testing. Clin Lab News, 36(8), 1-5.
 Contact: Salimetrics (USA)
Contact: Salimetrics (USA)