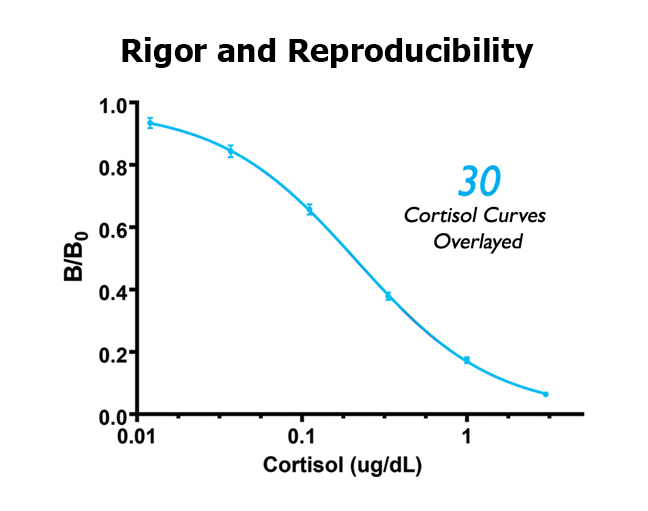
A good assay manufacturer will provide assays that meet rigorous quality acceptance criteria, and then provide protocols and guidance that ensures each laboratory can achieve great results. It’s a simple fact: the tighter the acceptance criteria, the less variability in results; the wider the acceptance criteria, the greater the variability in results. Salimetrics’ assays are manufactured and quality tested to rigorous acceptance criteria, and the Salimetrics Core Lab reliably achieves these standards.
The Highest Quality BSL-2 Biospecimen Testing
Expect clinical grade accuracy at a higher levels for research studies that need exceptional precision and accuracy. The Salimetrics CoreLab+ has been an integral part of Salimetrics since the company was founded and has collectively assayed millions of saliva and dried blood spot samples. Biobehavioral research is better when it's backed by rigorous and reproducible storage, processing, and analysis methods.
Get accurate Saliva, Dried Blood Spots, and DNA Analysis for your study — all through a lab partner with transparent pricing and unparalleled support. If the guidance you seek needs to go beyond handling, collection, and testing and your research demands the distinct advantage of a knowledgeable scientific pioneer as a partner—we’ve got you covered.
Attributes of A High-Quality Bioscience Laboratory
#1 Routine calibration of lab equipment
High-quality labs have all of their lab equipment in a scheduled, routine calibration program. Calibration of lab equipment allows for reproducible measurements; conversely, uncalibrated equipment is one of the principle sources of lab error and almost always guarantees that the same samples tested across different labs will have a high inter-lab assay variability.
#2 Rigorous quality acceptance criteria for assay results
#3 Documented CV% acceptance/repeat criteria
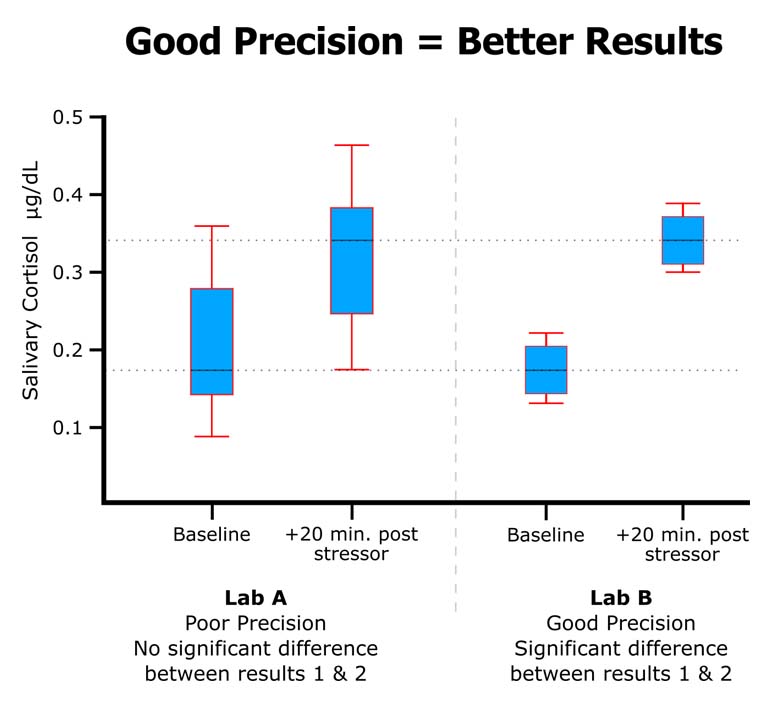
High-quality labs can control for many possible sources of lab error, but saliva samples and dried blood spots are unique. If the samples are not processed by the lab correctly, duplicate measurements of the same sample can have large deviations between the two measures. Consider duplicate measurements of the same sample having a CV% of 20%; this means that the two measurements are actually 40% apart, and the mean value is 20% apart from each of the two values. More often than not, only one of these measures is incorrect, so choosing the mean value suggests that the value intrinsically has a 20% error. Further, if the average CV% of the entire data set is 20%, then any conclusions made by the researcher would be unsupported if the conclusion did not rely on a >20% difference between the means.
In addition to following good protocols, a high-quality lab will avoid sample processing and handling errors by having well-documented criteria that only permits the acceptance of the duplicate mean based on a maximum allowable percentage of coefficient of variation between duplicate values, or when the absolute difference of the duplicate values is small and does not have biological significance. When the criteria are not met on a sample, the sample is rerun or excluded from the final data analysis. In addition, for quality control, the lab should record the sample as having been repeated.
#4 Experienced laboratory personnel trained in complex biospecimen testing to run the assays
Understanding how to work with viscous samples to avoid the introduction of bubbles and tiny volume discrepancies when pipetting, how to prevent bias across the plate, and even how to read and interpret the aforementioned quality control data, can make a difference in the integrity of the results. Both inter- and intra-assay CVs are improved when adequately trained personnel run assays. A high-quality lab will incorporate exercises such as pipetting proficiency into the training of personnel, and document the results, before actual assays are run.
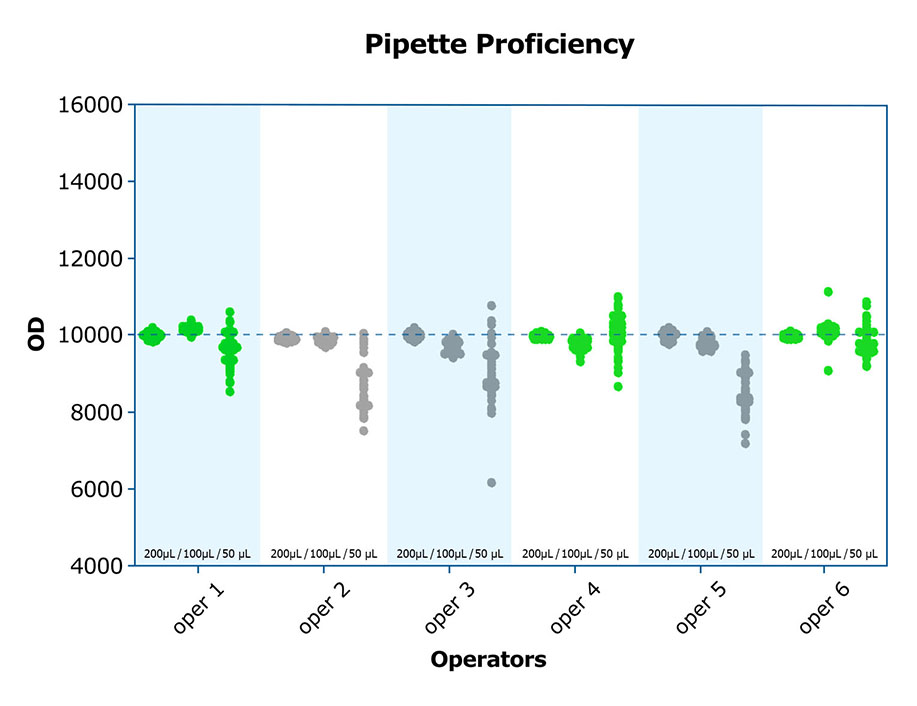
*Note: Operators #2, 3 and 5 should be retrained, focusing on small volume pipetting techniques.
#5 Routinely processes samples and performs testing
Practice makes perfect. The Salimetrics CoreLab+ has more expertise and experience at running assays compared to labs that run assays infrequently. It’s not that most labs are unable to get acceptable results from some of their biospecimen testing. It’s that most labs do not know how to get the best results from all of their biospecimen testing, and that’s simply because they have not tackled the full breadth of issues a proven, high-quality lab has experienced. The team behind the Salimetrics CoreLab+ knows how to troubleshoot and determine where potential issues may lie, and how to correct to get the best results.
#6 Participates in a proficiency testing program
Participating in a proficiency testing program is like getting a graded report card for the lab. It’s conducted after the lab meets all of the basic requirements for rigor, including training, and thereafter at minimum on an annual basis. For diagnostic labs, it is a regulatory requirement that they demonstrate proficiency in sample handling and processing by providing sample measurements on unknown, “controlled” samples. While this might not seem relevant to research, it is! A key finding from proficiency testing is that researchers can trust that the results because the results are repeatable.
#7 Uses and recommends validated biospecimen collection methods
High-quality labs know that how a sample is collected and handled prior to testing can significantly impact the measurements obtained from that sample. They understand that data from poorly handled samples will likely harm the data set when developing scientific associations and conclusions. For example, not correcting for flow rate when measuring an analyte that is impacted by flow, using an incorrect collection or sample transport method, using samples subjected to multiple freeze-thaws, or using an unvalidated materials for the collection of samples without fully understanding the bias/interference created by the material (See Part 2, How Good are Your Saliva Samples?) are all errors that may affect scientific conclusions. Conversely, following validated methods reduces variability: from collection; from transport; from storage; from freezing; from the bench – all of which increase the repeatability of the results. A high-quality lab knows what the validated collection methods are, and like Salimetrics, they know how to make the best sample collection recommendations for a specific study. These labs keep up with evolving best practices and are a great resource for newer researchers.
EASY & ACCURATE
Research Panel and Profiles
Salivary Cytokine Panel
| Target Analyte(s): |
Salivary Interleukin-1 Beta Salivary Interleukin-5 Salivary Interleukin-6 Salivary Interleukin-7 Salivary Interleukin-8 Salivary TNF-Alpha Salivary Interferon Gamma Salivary Interleukin-2 Salivary Interleukin-10 Salivary Interleukin-12p70 Salivary Interleukin-13 Salivary Interleukin-17A |
| Optimum Collection Volume: | 100 μL |
| Sample Test Volume: | 25 μL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | Multiple |
| Assay Range: | Multiple |
| Serum-Saliva Correlation: | N/A |
Salivary DLMO Profile
| Target Analyte(s): |
Salivary Melatonin |
| Optimum Collection Volume: | 500 μL/sample |
| Sample Test Volume: | 100 μL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 1.37 pg/mL |
| Assay Range: | 0.78-50 pg/mL |
Salivary SARS-CoV-2 (IgG) Antibody Panel
| Target Analyte(s): |
Salivary SARS-CoV-2 (N) IgG SARS-CoV-2 Spike Protein (S1) SARS-CoV-2 Nucleocapsid Protein (N) SARS-CoV-2 Receptor Binding Domain (RBD) |
| Optimum Collection Volume: | 0.3 - 1 mL |
| Sample Test Volume: | 25 μL |
| Collect With: |
Passive Drool Participant Sampling Packs Swab Method Participant Sampling Packs Children's Swab (SCS) Sampling Packs Infant's Swab (SIS) Sampling Packs |
| Assay Sensitivity: | Multiple |
| Assay Range: | N/A |
DBS Cytokine Panel 3-plex (IL-6, IL-10, TNF-alpha)
| Target Analyte(s): |
IL-6 IL-10 TNF-Alpha |
| Optimum Collection Volume: | 4 Spots |
| Sample Test Volume: | 3 Spots |
| Collect With: |
Dried Blood Spot Participant Sampling Pack |
| Assay Sensitivity: | Multiple |
| Assay Range: | Multiple |
| DBS-Plasma Correlation | Varies |
Salivary Analytes
Salivary 17 Alpha-OHP
| Optimum Collection Volume: | 125 μL |
| Sample Test Volume: | 50 μL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 3 pg/mL |
| Assay Range: | 5.1 pg/mL - 500pg/mL |
| Serum-Saliva Correlation: | 0.64 |
Salivary Aldosterone
| Optimum Collection Volume: | 175 μL |
| Sample Test Volume: | 100 µl |
| Collect With: |
Swab Method Participant Sampling Packs Children's Swab (SCS) Sampling Packs Infant's Swab (SIS) Sampling Packs Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 35 pg/mL |
| Assay Range: | ----- |
| Serum-Saliva Correlation: | 0.75-0.96 |
Salivary Alpha-Amylase
| Optimum Collection Volume: | 25 μL |
| Sample Test Volume: | 10 µL of saliva then 8 µL of X200 dilution |
| Collect With: |
Passive Drool Participant Sampling Packs Swab Method Participant Sampling Packs Children's Swab (SCS) Sampling Packs Infant's Swab (SIS) Sampling Packs |
| Assay Sensitivity: | 0.4 U/mL |
| Assay Range: | 2-400 U/mL |
| Serum-Saliva Correlation: | NA |
Salivary Androstenedione
| Optimum Collection Volume: | 125 μL |
| Sample Test Volume: | 50 µL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 5 pg/mL |
| Assay Range: | 10 pg/mL - 2430 pg/mL |
| Serum-Saliva Correlation: | NA |
Salivary C-Reactive Protein
| Optimum Collection Volume: | 225 μL |
| Sample Test Volume: | 150 µL of saliva, 100 µL of x2 dilution |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 1.79 pg/mL |
| Assay Range: | 25 pg/mL - 1600 pg/mL |
| Serum-Saliva Correlation: | NA |
Salivary Cortisol
| Optimum Collection Volume: | 75 uL |
| Sample Test Volume: | 25 μL |
| Collect With: |
Passive Drool Participant Sampling Packs Swab Method Participant Sampling Packs Children's Swab (SCS) Sampling Packs Infant's Swab (SIS) Sampling Packs |
| Assay Sensitivity: | <0.007 ug/dL |
| Assay Range: | 0.012-3.000 ug/dL |
| Serum-Saliva Correlation: | 0.91 |
Salivary Cotinine
| Optimum Collection Volume: | 75 µL |
| Sample Test Volume: | 20 µL |
| Collect With: |
Passive Drool Participant Sampling Packs Swab Method Participant Sampling Packs Children's Swab (SCS) Sampling Packs Infant's Swab (SIS) Sampling Packs |
| Assay Sensitivity: | 0.15 ng/mL |
| Assay Range: | 0.8 ng/mL - 200 ng/mL |
| Serum-Saliva Correlation: | NA |
Salivary DHEA
| Optimum Collection Volume: | 125 μL |
| Sample Test Volume: | 50 µL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 5 pg/mL |
| Assay Range: | 10.2 pg/mL - 1000 pg/mL |
| Serum-Saliva Correlation: | 0.86 |
Salivary DHEA-S
| Optimum Collection Volume: | 225 μL |
| Sample Test Volume: | 100 µL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 95.14 pg/mL |
| Assay Range: | 188.9 pg/mL- 15,300 pg/mL |
| Serum-Saliva Correlation: | NA |
Salivary Estradiol
| Optimum Collection Volume: | 225 μL* |
| Sample Test Volume: | 100 µL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 0.1 pg/mL |
| Assay Range: | 1 pg/mL - 32 pg/mL |
| Serum-Saliva Correlation: | 0.80 |
Salivary Estriol
| Optimum Collection Volume: | 175 μL |
| Sample Test Volume: | 100 µL of X2 dilution |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: |
16 pg/mL 1 pg/mL (HS) |
| Assay Range: |
20 pg/mL - 4860 pg/mL 5 pg/mL - 1215 pg/mL (HS) |
| Serum-Saliva Correlation: | 0.87 |
Salivary Estrone
| Optimum Collection Volume: | 255 μL |
| Sample Test Volume: | 100 µL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 3.04 pg/mL |
| Assay Range: | 3.1 pg/mL - 300 pg/mL |
| Serum-Saliva Correlation: | NA |
Salivary IgG
| Optimum Collection Volume: | 50 μL |
| Sample Test Volume: | 10 uL |
| Collect With: |
Passive Drool Participant Sampling Packs Swab Method Participant Sampling Packs Children's Swab (SCS) Sampling Packs Infant's Swab (SIS) Sampling Packs |
| Assay Sensitivity: | 0.043 ng/mL |
| Assay Range: | 0.3125 - 20 ng/mL |
| Serum-Saliva Correlation: | NA |
Salivary IgM
| Optimum Collection Volume: | 50 μL |
| Sample Test Volume: | 10 uL |
| Collect With: |
Passive Drool Participant Sampling Packs Swab Method Participant Sampling Packs Children's Swab (SCS) Sampling Packs Infant's Swab (SIS) Sampling Packs |
| Assay Sensitivity: | 0.03 ng/mL |
| Assay Range: | 0.39 – 25 ng/mL |
| Serum-Saliva Correlation: | NA |
Salivary Insulin
| Optimum Collection Volume: | 100 μL |
| Sample Test Volume: | 25 μL |
| Collect With: |
Passive Drool Participant Sampling Packs Swab Method Participant Sampling Packs Children's Swab (SCS) Sampling Packs Infant's Swab (SIS) Sampling Packs |
| Assay Sensitivity: | 0.39 µIU/mL |
| Assay Range: | 0.39 – 300 µIU/mL |
| Serum-Saliva Correlation: | 0.92 |
Salivary Interferon Gamma
| Optimum Collection Volume: | 100 μL |
| Sample Test Volume: | 25 μL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 0.37 pg/mL |
| Assay Range: | 0.37 – 4760 pg/mL |
| Serum-Saliva Correlation: | NA |
Salivary Interleukin-1 Beta
| Optimum Collection Volume: | 50 μL |
| Sample Test Volume: | 20 μL |
| Collect With: |
Passive Drool Participant Sampling Packs Swab Method Participant Sampling Packs Children's Swab (SCS) Sampling Packs Infant's Swab (SIS) Sampling Packs |
| Assay Sensitivity: | < 0.37 pg/mL |
| Assay Range: | 3.13 pg/mL - 200 pg/mL |
| Serum-Saliva Correlation: | NA |
Salivary Interleukin-10
| Optimum Collection Volume: | 100 μL |
| Sample Test Volume: | 25 μL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 0.04 pg/mL |
| Assay Range: | 0.04 – 1420 pg/m |
| Serum-Saliva Correlation: | NA |
Salivary Interleukin-12p70
| Optimum Collection Volume: | 100 μL |
| Sample Test Volume: | 25 μL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 0.11 pg/mL |
| Assay Range: | 0.11 – 2180 pg/mL |
Salivary Interleukin-13
| Optimum Collection Volume: | 100 μL |
| Sample Test Volume: | 25 μL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 0.24 pg/mL |
| Assay Range: | 0.24 – 2072 pg/mL |
| Serum-Saliva Correlation: | NA |
Salivary Interleukin-2
| Optimum Collection Volume: | 100 μL |
| Sample Test Volume: | 25 μL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 0.09 pg/mL |
| Assay Range: | 0.09 – 6040 pg/mL |
Salivary Interleukin-6
| Optimum Collection Volume: | 135 μL |
| Sample Test Volume: | 60 μL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 0.07 pg/mL |
| Assay Range: | 1.56 – 100 pg/mL |
| Serum-Saliva Correlation: | NA |
Salivary Interleukin-8
| Optimum Collection Volume: | 100 μL |
| Sample Test Volume: | 25 μL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 0.07 pg/mL |
| Assay Range: | 0.07 – 2336 pg/mL |
| Serum-Saliva Correlation: | NA |
Salivary Melatonin
| Optimum Collection Volume: | 225 μL |
| Sample Test Volume: | 100 µL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 1.35 pg/mL |
| Assay Range: | 0.78-50 pg/mL |
| Serum-Saliva Correlation: | 0.81 |
Salivary Osteocalcin
| Optimum Collection Volume: | 150 μL |
| Sample Test Volume: | 25 μL |
| Collect With: |
Passive Drool Participant Sampling Packs Swab Method Participant Sampling Packs Children's Swab (SCS) Sampling Packs Infant's Swab (SIS) Sampling Packs |
| Assay Sensitivity: | 13.72 pg/mL |
| Assay Range: | 9.77 - 10,000 pg/mL |
| Serum-Saliva Correlation: | N/A |
Salivary Oxytocin
| Optimum Collection Volume: | 150 μL |
| Sample Test Volume: | 25 μL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 4 pg/mL |
| Assay Range: | 6.4-1000 pg/mL |
| Serum-Saliva Correlation: | N/A |
Salivary Progesterone
| Optimum Collection Volume: | 125 μL |
| Sample Test Volume: | 50 μL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 5 pg/mL |
| Assay Range: | 10 pg/mL - 2430 pg/mL |
| Serum-Saliva Correlation: | NA |
Salivary SARS-CoV-2 (N) IgG
| Optimum Collection Volume: | 200 μL |
| Sample Test Volume: | 50 uL |
| Collect With: |
Passive Drool Participant Sampling Packs Swab Method Participant Sampling Packs Children's Swab (SCS) Sampling Packs Infant's Swab (SIS) Sampling Packs |
| Assay Sensitivity: | 92% |
| Assay Sensitivity: | 98% |
| Assay Range: | Qualitative |
| Serum-Saliva Correlation: | NA |
Salivary SIgA
| Optimum Collection Volume: | 50 μL |
| Sample Test Volume: | 25 μL |
| Collect With: |
Passive Drool Participant Sampling Packs Swab Method Participant Sampling Packs Children's Swab (SCS) Sampling Packs Infant's Swab (SIS) Sampling Packs |
| Assay Sensitivity: | 2.5 μg/mL |
| Assay Range: | 2.50 µg/mL – 600 µg/mL |
Salivary Testosterone
| Optimum Collection Volume: | 75 μL |
| Sample Test Volume: | 25 μL |
| Collect With: |
Passive Drool Participant Sampling Packs Swab Method Participant Sampling Packs Children's Swab (SCS) Sampling Packs Infant's Swab (SIS) Sampling Packs |
| Assay Sensitivity: | 1 pg/mL |
| Assay Range: | 6.1 pg/mL - 600 pg/mL |
| Serum-Saliva Correlation: | 0.96 |
Salivary TNF-Alpha
| Optimum Collection Volume: | 425 μL |
| Sample Test Volume: | 200 μL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 0.106 pg/mL |
| Assay Range: | 0.04 - 1360 pg/mL |
| Serum-Saliva Correlation: | NA |
Salivary Total Protein
| Optimum Collection Volume: | 75 μL |
| Sample Test Volume: | 25 μL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 5 μg/mL |
| Assay Range: | ----- |
| Serum-Saliva Correlation: | NA |
Salivary Transferrin
| Optimum Collection Volume: | 75 μL |
| Sample Test Volume: | 20 µL |
| Collect With: |
Passive Drool Participant Sampling Packs |
| Assay Sensitivity: | 0.08 mg/dL |
| Assay Range: | 0.08 mg/dL - 6.6 mg/dL |
| Serum-Saliva Correlation: | NA |
Salivary Uric Acid
| Optimum Collection Volume: | 25 μL |
| Sample Test Volume: | 10 µL |
| Collect With: |
Passive Drool Participant Sampling Packs Swab Method Participant Sampling Packs Children's Swab (SCS) Sampling Packs Infant's Swab (SIS) Sampling Packs |
| Assay Sensitivity: | 0.07 mg/dL |
| Assay Range: | 0.07- 20 mg/dL |
| Serum-Saliva Correlation: | 0.84 |
Biomarkers in Dried Blood Spots
DBS HbA1c
| Optimum Collection Volume: | 3 Spots |
| Sample Test Volume: | 1 Spot |
| Collect With: |
Dried Blood Spot Participant Sampling Pack |
| Assay Sensitivity: | 3.9% |
| Assay Range: | 3.9–18.8% |
| DBS-Whole Blood Correlation: | 0.93 |
DBS C-Reactive Protein
| Optimum Collection Volume: | 3 Spots |
| Sample Test Volume: | 1 Spot |
| Collect With: |
Dried Blood Spot Participant Sampling Pack |
| Assay Sensitivity: | 0.006 mg/L |
| Assay Range: | 0.006 –20 mg/mL |
| DBS-Plasma Correlation: | 0.98 |
Add DNA Analysis to My Study
Considerations for adding Salivary DNA to analyte Studies:
You can combine salivary analytes with easy, accurate, and affordable genomic testing using Salimetrics SalivaLab and the same sample that you are already collecting – no specialized saliva collection devices or additional samples are required.
Don’t know what SNPs are right for you? The SalivaLab’s DNA team specializes in genetic testing services, we recommend you Request a DNA Consult (gratis) to learn more about common considerations such as # of samples, participant ethnicity, and IRB Approval.
All DNA Services
DNA Extraction and Normalization
Single Nucleotide Polymorphism (SNP) Genotyping
VNTR & STR Analysis
Clinical and Diagnostics

Industry-Leading Clinical Research Services for Biomarker Integration
The Salimetrics CoreLab+ is a CLIA certified lab which has been supporting the drug discovery & development process of pharmaceutical and contract research organizations for over ten years. Salimetrics has standardized the tools necessary to provide cost-effective, biological measurements for saliva and dried blood spot biomarkers and analytes in your biomedical, pharmacological, or clinical research project. The Salimetrics CoreLab+ provides unparalleled sample analysis in support of your biomarker needs or therapeutic area of expertise.
Our Approach
The Salimetrics platform gives you flexibility for incorporating biomarkers into your drug development or clinical research program. From early stage drug discovery, target validation, non-clinical/pre-clinical pharmacokinetic/pharmacodynamic studies, and through all phases of clinical trials, Salimetrics can provide your team with the highest-quality biospecimen data. Our research team can assist clinical investigators in designing, participant screening or therapeutic drug monitoring to achieve your investigational research goals.
Getting Started
If you’re interested in incorporating validated biomarkers via saliva or dried blood spots into your drug discovery program, the fastest way to reach us is to schedule a consultation by calling us at: 800.790.2258 or Ask An Expert
Both large and small drug discovery projects can benefit from enhanced biomarker assay services including:
- Priority Testing
- Flexibility to meet changing needs
- Validation Assistance
- Specialized collection and assay training
- Responsive Project Specialist for study-specific inquiries
- Logistical management
- Secure network specific File Transfer Protocol (FTP) for data
- Specimen shipping/ tracking
- Custom collection supplies
- Assistance with project design
 Contact: Salimetrics (USA)
Contact: Salimetrics (USA)