(Aug 1, 2019)
The OnTimePoint™ Saliva Collection Management System for Salivary Bioscience is now in development as part of Salimetrics’ core mission to ensure researchers and clinicians have the better methods that lead to increased rigor and reproducibility in their Salivary Bioscience.
Today, in order to increase the level of rigor and reproducibility in their Salivary Bioscience, researchers are faced with the difficult task of maintaining oversight of their study participants’ compliance to saliva collection protocols. Soon, Salimetrics’ new OnTimePoint™ Saliva Collection Management System will make that oversight simpler. “OnTimePoint™ makes it possible to know in real-time the exact time your study samples were collected, and gives you, the researcher, the opportunity to see what’s going on and do something about it. Now you have the tools to make sure your participants and their samples are 100% compliant with their saliva collection,” says Supriya Gaitonde, Ph.D., Salimetrics Senior Applications Scientist and member of the development team. Dr. Gaitonde works with hundreds of researchers in support of strengthening the methods used in their salivary bioscience studies.
Recently, the NIH and several scientific review articles have encouraged researchers to utilize tools and methods that will aid in monitoring compliance of their study participant’s collection regimen. However, finding and tailoring existing tools to salivary bioscience studies presents logistical challenges. Many tools for researchers are expensive and impractical, require additional IRB or regulatory governance, or present to participants and coordinators as being overly complex, unsupported, or simply incompatible with the study design logistics. None of these existing tools were designed to leverage the better methods of Salivary Bioscience that are fundamental in meeting the researcher’s study requirements.
“This “needs gap” – a practical, inexpensive, easy to use, easy to coordinate, easy to implement, easy to monitor Saliva Collection Management System that leverages the better methods of Salivary Bioscience – was the catalyst for the Salimetrics’ team.” Says Dr. Gaitonde. “We are a company dedicated to details, constantly in pursuit of scientific knowledge that creates the better methods for the salivary bioscience community, so we created OnTimePoint™. Because Salimetrics’ breadth of knowledge reaches more than 5,000 salivary bioscience studies each year, we know that our team is uniquely qualified to develop a solution that applies better methods to capture datapoints with a higher degree of accuracy.”
Traditional studies require research coordinators to spend large portions of their time scheduling, tracking, logging and coordinating saliva collections. This new saliva collection management system provides a boost to efficiency. By utilizing mobile and cloud-based technology to automate much of the sample collection logistics, OnTimePoint™ can reduce coordinator time spent on these tasks by more than 50%; coordinators can double their output and spend more time on other aspects, and as a result, increase the power of their study. “OnTimePoint™ is an extremely powerful tool that can add rigor and reproducibility to Salivary Bioscience, while at the same time, increase productivity and reduce the overall cost of the study,” says Chris Schwartz, Salimetrics’ Marketing Manager and another member of the OnTimePoint™ development team.
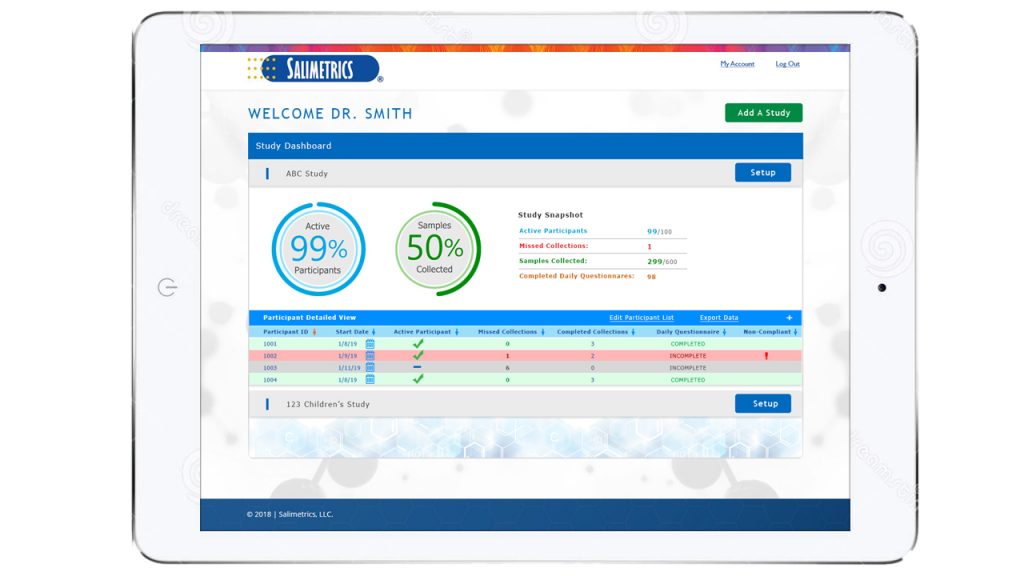
The OnTimePoint™ Saliva Collection Management System license is purchased on a study-by-study basis for a flat fee. With an active account, investigators can setup and launch their study in real-time via a computer or tablet. Participants then receive instructions, questionnaires, and sample collection notifications on a smartphone through the OnTimePoint™ app, compatible with both Android and iOS platforms. Designed exclusively for use with the saliva collection supplies available through Salimetrics, OnTimePoint™ manages the research workflow from receipt of collection supplies by the researcher, to when samples are tested by the Salimetrics’ SalivaLab, to the receipt of final test data. Using advanced algorithms, OnTimePoint™ tracks the status of the sample, provides notifications and feedback on sample collection timepoints (including EMAs, questionnaires, participant engagement), and can flag potentially non-compliant samples for repeat collection. OnTimePoint™ also offers a robust visual interface between the researcher and participant, making saliva collection and compliance oversight a simpler task.
“OnTimePoint™ is truly a better method for maintaining rigor and reproducibility that will meet the fundamental needs of Salivary Bioscience and maintain the standards required by the scientific community,” says Dr. Gaitonde.
To learn more about OnTimePoint or connect to a Salimetrics representative, investigators can visit the Salimetrics Website.
 Contact: Salimetrics (USA)
Contact: Salimetrics (USA)