(September 11, 2018)
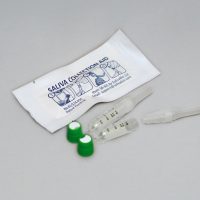
SalivaBio’s Saliva Collection Aid has become a worldwide industry standard in salivary bioscience for passive drool saliva collection.
The emergence of the Saliva Collection Aid in 2012 marked a milestone in the history of saliva collection devices. “Passive drool saliva collection has always been the better choice, so coming up with a way to improve the passive drool method is a story in itself,” says Douglas Granger, Ph.D., Salimetrics Founder and Chief Scientific and Strategy Advisor. “In the 80s and 90s, sample collection techniques were crude, idiosyncratic to each laboratory, and often designed to maximize the volume of sample collected without attention to how the protocols changed the integrity of the specimen. Early swab methods were designed for use when the major scientific focus was on salivary cortisol. However, the field of salivary bioscience has expanded the range of the tests it can perform well beyond salivary cortisol, and since swabs can be restrictive to what can be accurately measured in saliva, the best option for improving the integrity of the specimen was to improve and standardize the passive drool saliva collection method.”
In retrospect, many early saliva collection techniques in the published literature were causing interference with assay quality. “Substantive progress in advancing our understanding had to be put on pause while we carefully worked out the details of how to collect, handle, transport, and store saliva specimens,” says Dr. Granger. “We learned early on that some swab materials acted as a filter and interfered with the nature of the sample in ways that we could not predict. At the time, salivary research had not fully elucidated the biology of oral fluid. Passive drool was preferred by researchers because it represented a minimally manipulated sample.” However, in the beginning, there was no easy way to facilitate passive drool collection, which led to poor adoption by investigators in the salivary bioscience community. Passive drool was also difficult for some participants and no universally accepted device was available to easily facilitate the transfer of saliva from the mouth to a sample storage container.
In 2012, SalivaBio patented and released the Saliva Collection Aid, a device that enabled the collection of passive drool samples in a simple, universal manner that improved the integrity of the specimen and also improved participant compliance. “Since its release, the Saliva Collection Aid has become the method of choice. It is widely preferred by both researchers and participants,” says Dr. Granger. “From being heavily integrated into academic research to its establishment in functional lab testing and clinical trials, the introduction of the Saliva Collection Aid opened a wide window of research opportunities by no longer restricting what could be done with the saliva specimen.”
The Saliva Collection Aid’s simple design comfortably and easily guides the participant’s saliva through a canal and into common externally threaded cryovials. Vented side-ports reduce the production of bubbles and enable large volumes of saliva to be collected in a short amount of time. “It is an elegant, simple design in both form and function,” says Dr. Granger. “Presentation has a lot to do with acceptance and perception. SalivaBio’s dual focus on facilitating sample handling and storage means it’s efficient for the researcher, while easy, clean, and safe sample collection for the participant means better participant compliance.”
Many investigators consider saliva collection a novel decision, but research shows that sample collection has a tremendous impact on data quality. “It’s often a decision most researchers will make unknowingly, without considering the downstream implications to sample quality or processing efficiency,” says Supriya Gaitonde, Ph.D., Salimetrics Senior Applications Scientist. “For the two major decisions facing salivary bioscience researchers, we have this saying; “Collect Right, Test Right.” When you distill it down like this, it’s not complicated.
Read this article on PR Web
 Contact: Salimetrics (USA)
Contact: Salimetrics (USA)